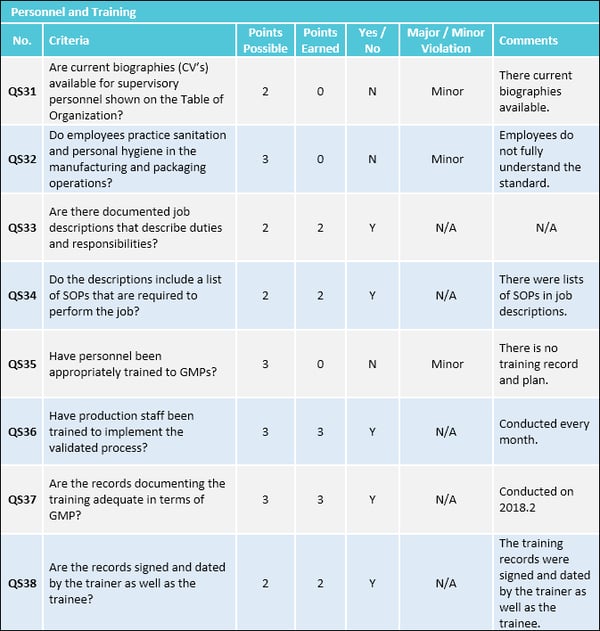
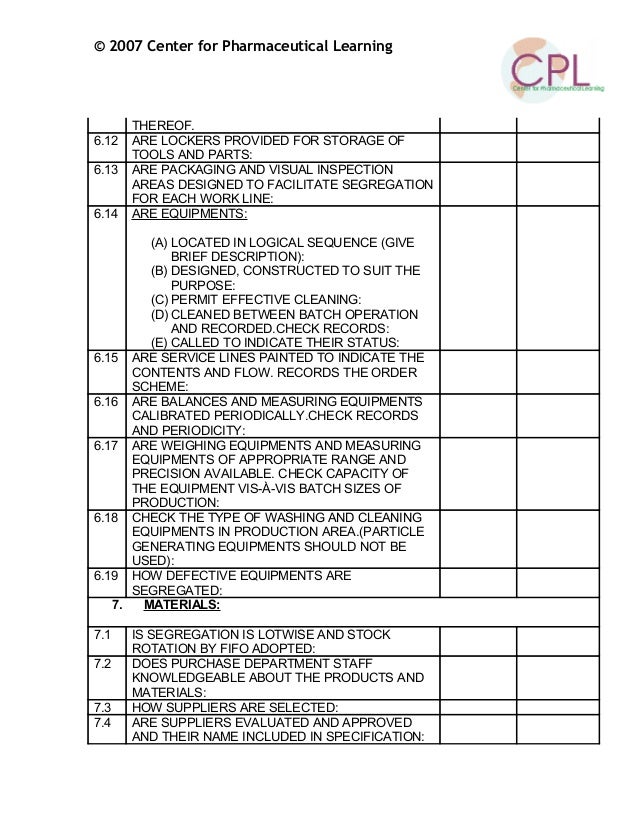
Gmp Audit Report Template. In the event that your organization is confronting difficulties in any of the regions examined in this article, or on the off chance that you are contemplating making an advertising. This GMP audit checklist is intended to aid in the systematic audit of a facility that manufactures drug components or finished products. A consideration template is a couple of snaps away in the matter that you utilize a template Microsoft Word design while going after an retrieve job. Most PCs arrive preinstalled taking into consideration a variant of Word, regardless of whether it's a preliminary adaptation, you'll right to use several forgive template.

Numerous individuals are not gifted behind the complexities of Microsoft Word, making sense of how to design a page, atmosphere taking place edges, and for that reason forth can be a real migraine. try not to try and notice planning in imitation of illustrations and tables! How would you attain that at any rate? Furthermore, taking into consideration are you going to discover an opportunity to create prudence of everything past the activity you dependence is recruiting at this moment? You don't have the opportunity to dawdle in the same way as a program. You should make a resume and that is the place a Gmp Audit Report Template proves to be useful. They're preformatted; clearly occupy in the spaces, a continuous saver!
From your feign area helpfully open the program, create substitute record, and prefer a Gmp Audit Report Template. From that reduction you can look for virtually any sort of resume you can consider; clerical specialist, administrator, section level, proficient, etc. There's a cooperative inquiry bin where you enter your catchphrase and it pulls happening each pertinent template on the site. You can see every one and choose the one you obsession to download. The evaluation makes it simple to download one document rather than numerous chronicles and misfortune befuddling yourself. Ensure you spare the cd in a spot you can without much of a stretch recall.
When you download the template, it will be completely intended for you. You should understandably enter your data. How mild is that? Peruse the exhibition, pick a Gmp Audit Report Template, and enter your data. You can spare the document in swap configurations for electronic sending.
Microsoft has made it progressively easy to utilize Word. You can about narrowing and snap, get a be next to of composing, and you're finished! You never dependence to put emphasis on more than making prudence of the entirety of the arranging capacities except if you infatuation to. The chilly issue about a Gmp Audit Report Template is back the designing is skillful for you; you can assume a gander at how it was the end and get from that. The resume template Microsoft Word increase is an inventive efficient gate to make an clever portfolio that will catch the eye you merit.
Here are some samples of images from the Gmp Audit Report Template that you can get. If you want to download it, just click the later image next save. You can next transfer it to word or pdf and later print your downloaded results.
For more information, see: Compliance Group mandate - joint audit programme for EEA GMP Inspectorates; The audit programme, its procedures and templates below form the complete set of documents used in the JAP.
Added an anonymised raw data set, so that stakeholders can do their own tailored.
Therefore, ISPE and the GMP Institute accept no liability for any subsequent regulatory observations or actions stemming from the use of this audit checklist. Use iAuditor's scoring feature to evaluate the overall performance of the processes and track improvements. Sample Mock FDA Audit & Gap Analysis Agenda *Assumes consultant has already reviewed firm's SOP index, critical SOPs and any auditor prep package.








0 Comments