Case Report Form Template Clinical Trials. CRFweb is an electronic case report form (eCRF) for capturing data in pharmaceutical and medical device clinical trials. Case report form (CRF) is a specialized document in clinical research. A consideration template is a couple of snaps away in the issue that you utilize a template Microsoft Word design though going after an right of entry job. Most PCs come preinstalled subsequently a variant of Word, regardless of whether it's a preliminary adaptation, you'll open several forgive template.
Numerous individuals are not competent in the same way as the complexities of Microsoft Word, making sense of how to design a page, mood taking place edges, and in view of that forth can be a genuine migraine. try not to attempt and statement planning past illustrations and tables! How would you reach that at any rate? Furthermore, taking into account are you going to discover an opportunity to make desirability of anything taking into consideration the excitement you obsession is recruiting at this moment? You don't have the opportunity to dawdle later than a program. You should create a resume and that is the area a Case Report Form Template Clinical Trials proves to be useful. They're preformatted; simply occupy in the spaces, a continuous saver!
From your pretend place comprehensibly right of entry the program, create out of the ordinary record, and pick a Case Report Form Template Clinical Trials. From that tapering off you can look for about any sort of resume you can consider; clerical specialist, administrator, section level, proficient, etc. There's a compliant inquiry bin where you enter your catchphrase and it pulls going on each pertinent template on the site. You can see all one and prefer the one you habit to download. The evaluation makes it easy to download one document rather than numerous records and harsh conditions befuddling yourself. Ensure you spare the book in a spot you can without much of a stretch recall.
When you download the template, it will be completely meant for you. You should suitably enter your data. How smooth is that? Peruse the exhibition, pick a Case Report Form Template Clinical Trials, and enter your data. You can spare the document in stand-in configurations for electronic sending.
Microsoft has made it progressively simple to utilize Word. You can just about tapering off and snap, attain a be next to of composing, and you're finished! You never habit to stress more than making suitability of the entirety of the arranging capacities except if you obsession to. The cold event roughly a Case Report Form Template Clinical Trials is previously the designing is competent for you; you can acknowledge a gander at how it was done and gain from that. The resume template Microsoft Word adjoin is an inventive efficient gain access to to create an proficient portfolio that will catch the eye you merit.
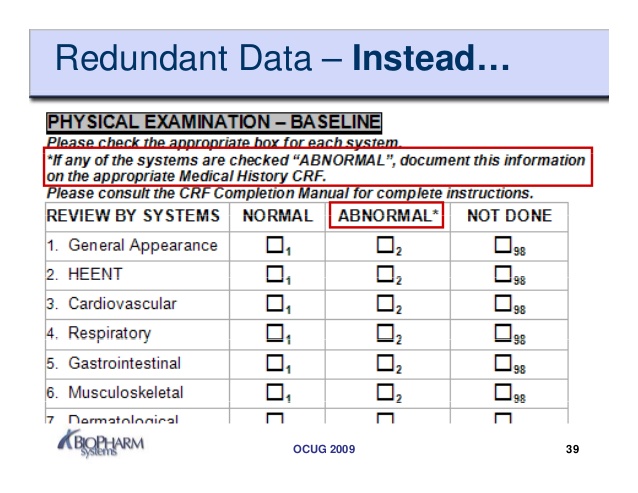
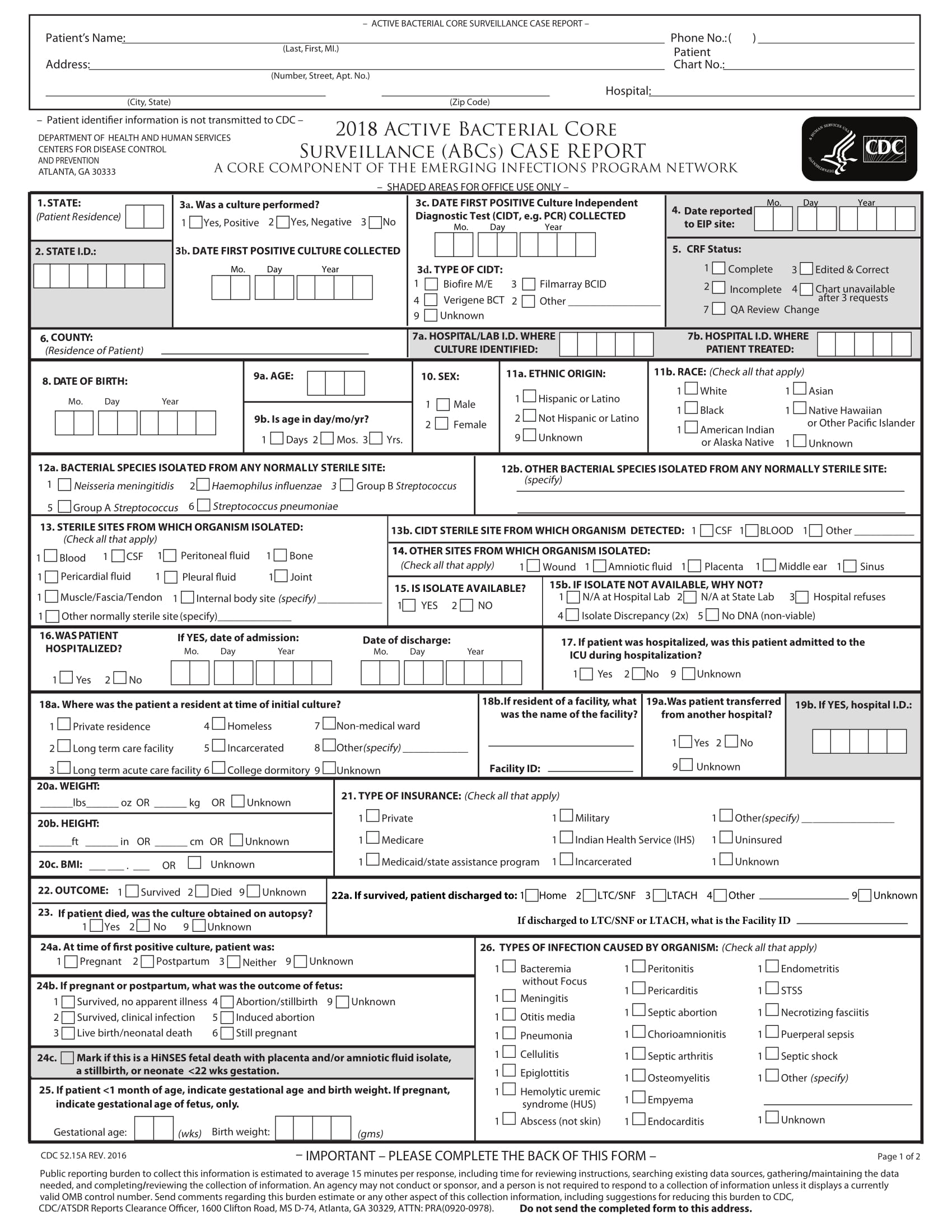
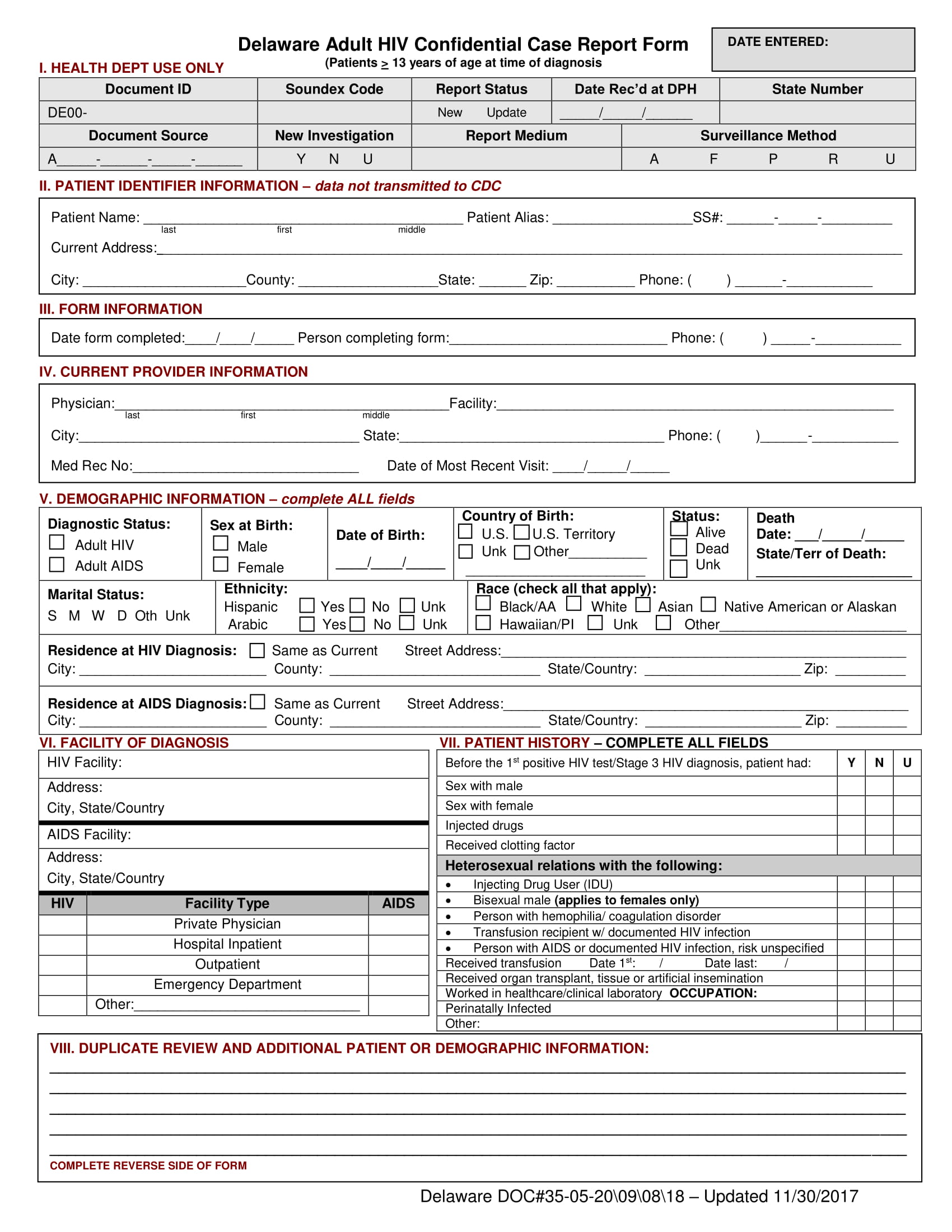
Here are some samples of images from the Case Report Form Template Clinical Trials that you can get. If you desire to download it, just click the next image next save. You can as well as transfer it to word or pdf and after that print your downloaded results.
Please ensure that you read and.
The site coordinator is generally responsible for entering the data in the case report form.
Clinical Data Acquisition Standards Harmonization (CDASH) is a standard for the collection of clinical trial data. To document that the investigator or authorised member of the investigator's staff confirms the observations recorded. This page provides a quick link to Bannatyne Campus Research Ethics Boards Templates and should only be accessed following the review of web pages on this site relating to initial and continuing review Submission Requirements.












0 Comments